In this application note we demonstrate a multi-residue UPLC-MS/MS method developed for the determination of 552 pesticides and relevant metabolites in fruits, vegetables, cereals and black tea extracts using ACQUITY UPLC I-Class coupled with Xevo TQ-XS
As the population grows, demand for food consumption and global trade in the food industry has also increased. Hundreds of pesticides are routinely used for crop protection across the globe, traces of pesticides left in treated commodities are called “residues”. Regulations are in place for Maximum Residue Levels (MRL), that are legally tolerated in or on food and feed when pesticides are applied correctly in accordance with Good Agricultural Practice. A growing target list of pesticides in complex matrices, and the need for low limits of detection, bring various challenges for multi-residue methods.
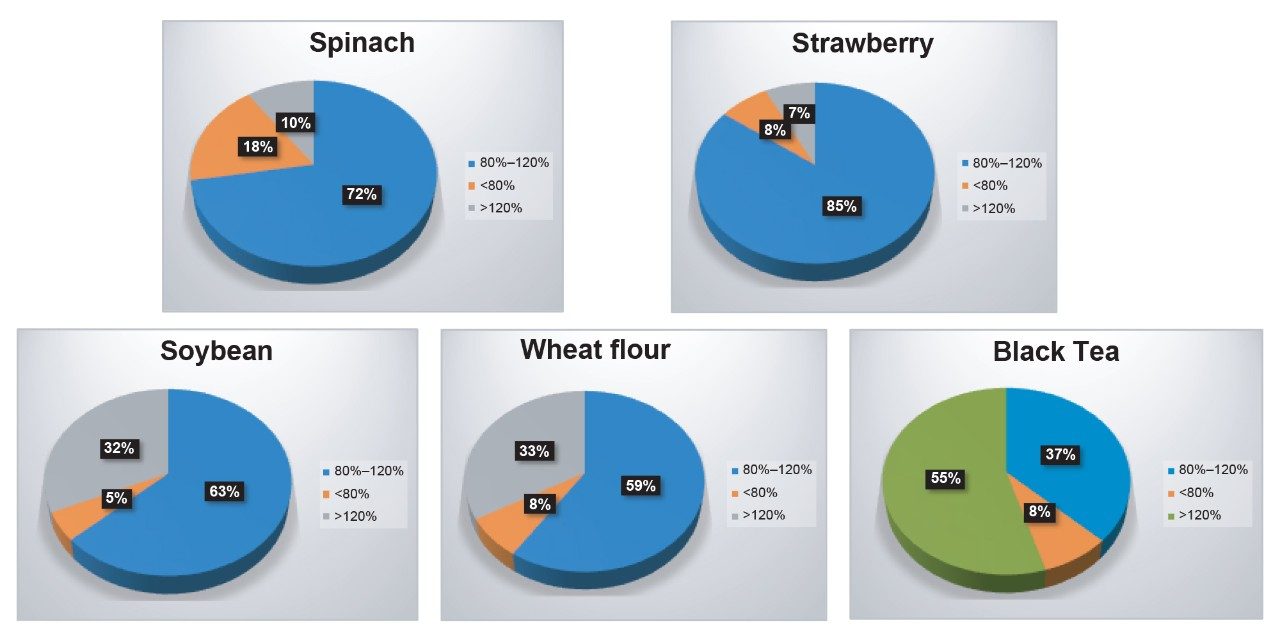
A multi-residue method for 552 pesticides and relevant metabolites was developed for various food commodities. Extracts from representative commodities, including high-water content (spinach), high acid and highwater content (strawberry), high oil and very low-water content (soybean), high protein and low-water and fat content (wheat flour), and difficult or unique commodities (black tea) were chosen to assess the performance of the UPLC-MS/MS method.
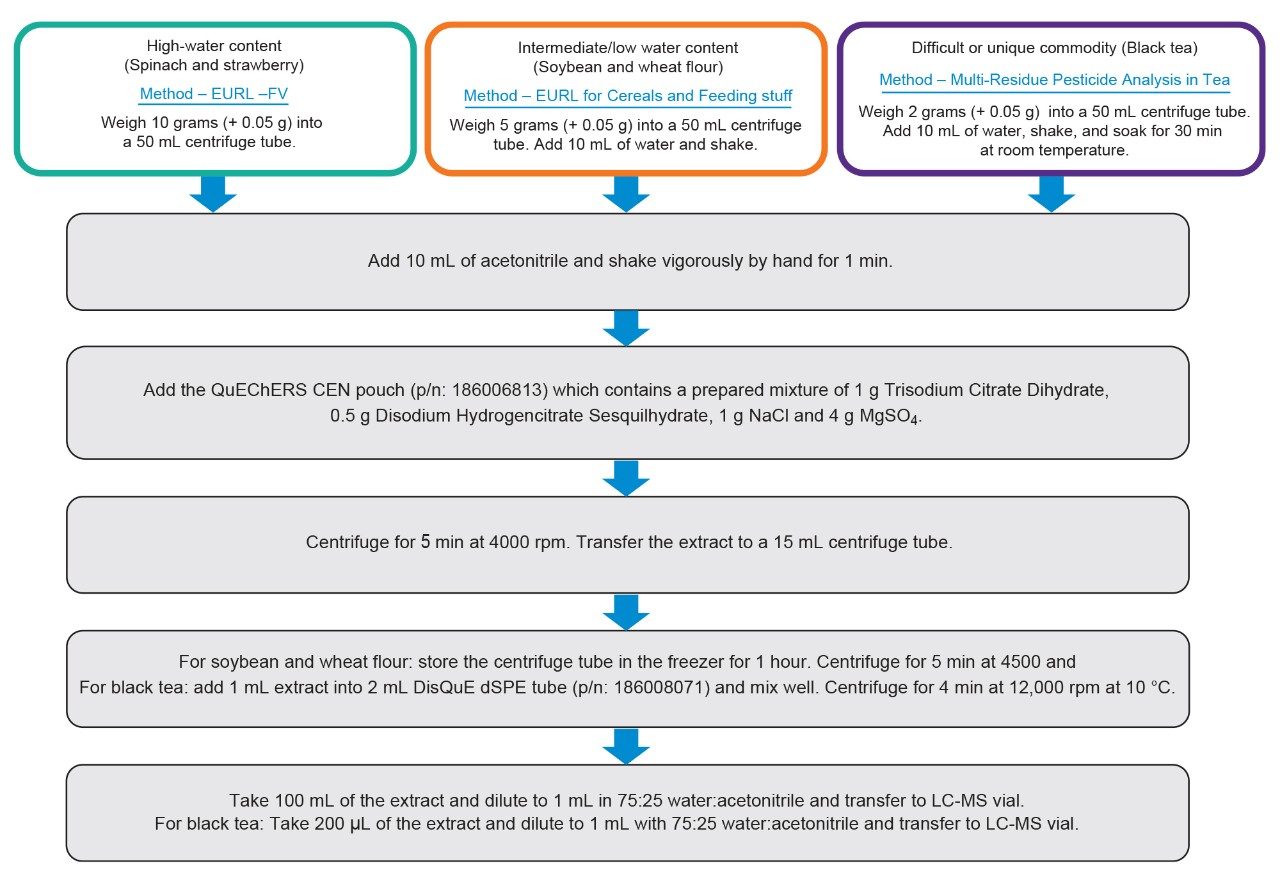
Representative samples from different commodity groups were chosen including high-water content (spinach), high acid and high-water content (strawberry), high oil and very low-water content (soybean), high protein and low water and fat content (wheat flour), and difficult or unique commodities (black tea). These samples were purchased from a local retail store. The samples were immediately homogenized in a food processor and frozen until extraction was performed. The samples were extracted using the QuEChERS CEN method. For high aqueous samples, such as spinach and strawberry, samples were prepared according to the European Union Reference Laboratory (EURL) fruits and vegetable method.1 For intermediate or low water content, such as soybean and wheat flour, the EURL method for cereals and feeding stuff was used.2 For low water and high carbohydrates, such as tea, the QuEChERS extraction with sample cleanup method was followed.3
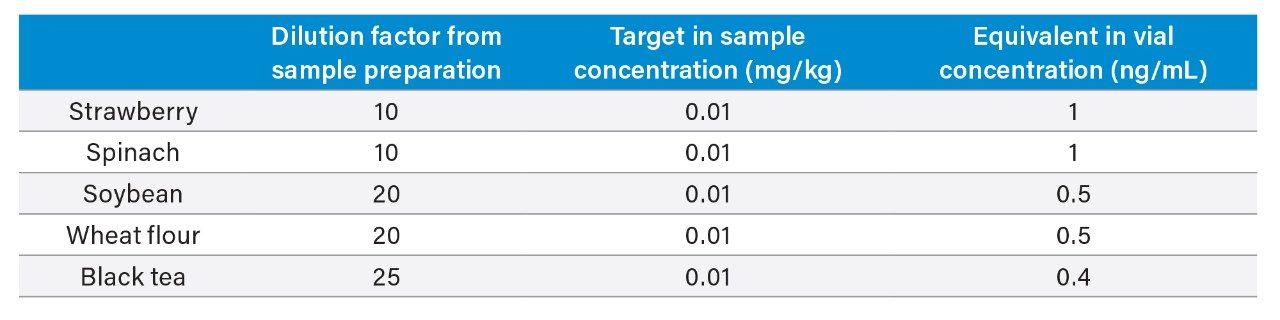
The performance of the UPLC-MS/MS method was assessed by spiking a mixture of 256 pesticides (see Annex for details) into extracts of spinach, strawberry, soybean, wheat flour and black tea at eight concentrations between 0.0001 to 0.1 mg/kg. Matrix-matched calibration standards were injected in a way that simulated the analysis of a batch of samples; calibration standards bracketed a series of replicate injections (n=6) at three matrix-matched levels.
|
UPLC system: |
ACQUITY UPLC I-Class with FL Sample Manager |
|
Column: |
ACQUITY UPLC HSS T3 Column, 1.8 μm, 2.1 × 100 mm (186003539) |
|
Post injector mixing kit: |
50 μL extension loop (430002012) |
|
Mobile phase A: |
5 mM ammonium formate in water + 0.1% formic acid |
|
Mobile phase B: |
5 mM ammonium formate in 50:50 MeCN: MeOH + 0.1% formic acid |
|
Injection volume: |
5 μL (PLNO mode) |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Run time: |
19 minutes |
|
Data acquisition and processing: |
MassLynx 4.2 and TargetLynx XS |
|
MS instrument: |
Xevo TQ-XS |
|
Ionization: |
Electrospray |
|
Polarity: |
Positive and negative ion mode |
|
Capillary voltage: |
+2.0/-2.0 kV |
|
Desolvation temp.: |
300 °C |
|
Desolvation gas flow: |
1000 L/Hr |
|
Source temp.: |
150 °C |
|
Cone gas flow: |
150 L/Hr |
|
Strong needle wash (SNW): |
0.1% formic acid in acetonitrile |
|
Weak needle wash (WNW): |
Mobile phase A |
|
Seal wash: |
10% methanol in water |
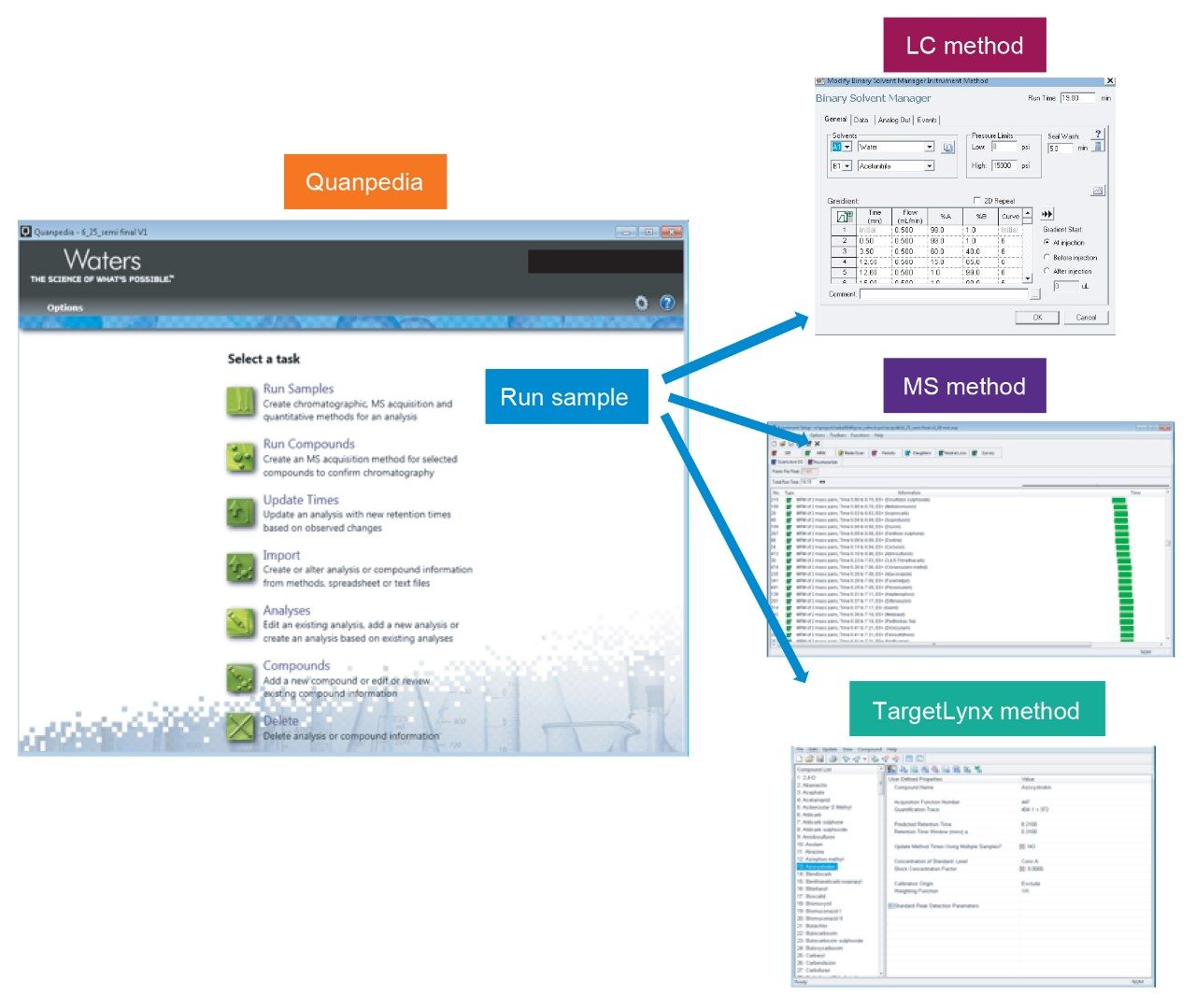
The LC-MS/MS method for 552 pesticides was created using the Quanpedia database. The Quanpedia database automatically creates LC and MS acquisition methods as well as processing methods from a compendium of compound specific MS parameters such as transitions, cone voltage, and collision energy. The MS method used for this work contains at least 2 MRM transitions per pesticide resulting in a method with more than 1000 MRMs. The auto-dwell functionality in the MS method managed the dwell time for each transition, ensuring the required number of data points (10–14) is available across each peak. Figure 2 shows an example of method creation in Quanpedia for multi-residue analysis.
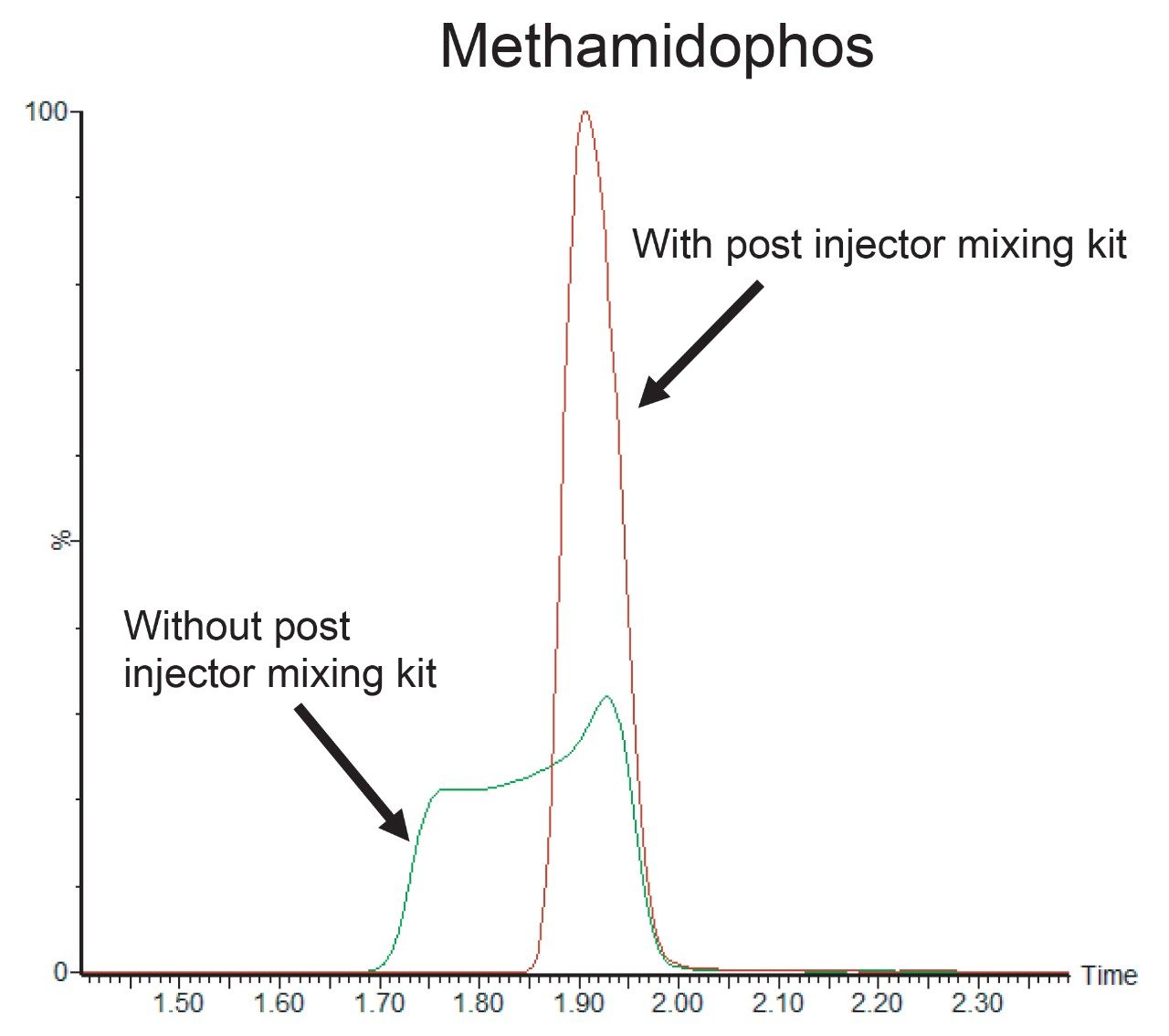
This multi-residue method was developed for a wide range of pesticides with varying chemical properties. A few of these pesticides, such as methamidophos and acephate, are very polar and elute early in the chromatogram. Injecting samples containing a moderate organic content (25%) often results in fronting and/or splitting peaks for the early eluting compounds. Reducing organic content in the sample diluent prior to its injection onto the column may help to improve the peak shape of early eluting analytes. Installing a post injector mixing kit, between the injector port and column, allows the injection of typical QuEChERS extracts into high aqueous mobile phase without compromising peak shape. Before making the injection, the extension loop is filled with the high aqueous mobile phase, which provides more volume to aid dispersion of the sample into the aqueous solvent prior to transfer onto the column. In this way, a moderate level (25%) of organic solvent can still be used to prepare the sample, in which most of the pesticides remain soluble, whilst still providing good peak shape for the very polar analytes. Figure 3 shows the peak shape of methamidophos with and without the post injector mixing kit; good peak shape is observed with the extension loop fitted, which provided more reliable quantitation and greater sensitivity and hence a lower limit of detection (LOD).
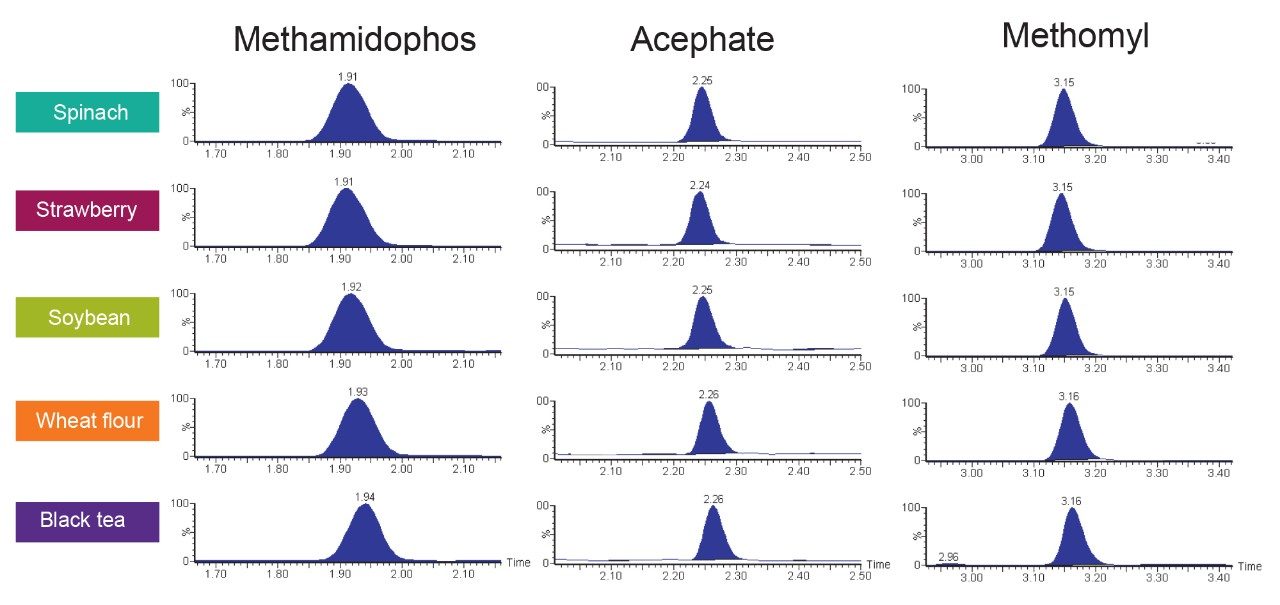
As shown in Figure 4, the retention time of methamidophos is obtained at ~1.9 minutes with a peak width at the base line of ~7 seconds in all studied matrices. The retention time of methamidophos is greater than two times the retention time corresponding to the void volume of the column (void time 0.46 min).
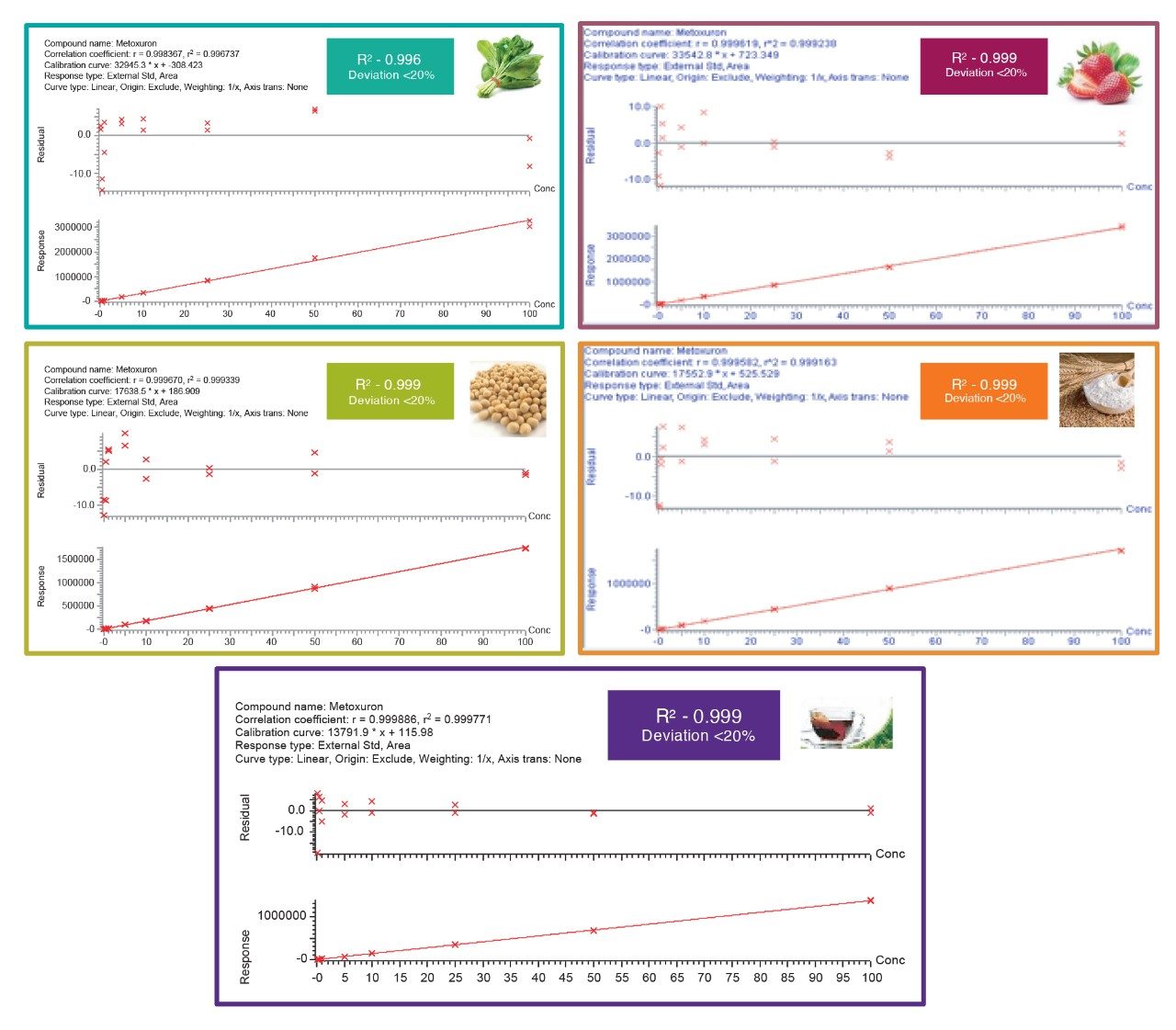
The calibration graphs for the majority of the 256 compounds in all the matrices showed values for coefficient of determination (R2) greater than 0.99 and back-calculated concentrations (residuals) were all within the ± 20% SANTE tolerance.4 Figures 5 shows matrix matched calibration graphs for metoxuron, a representative analyte, in five studied matrices.
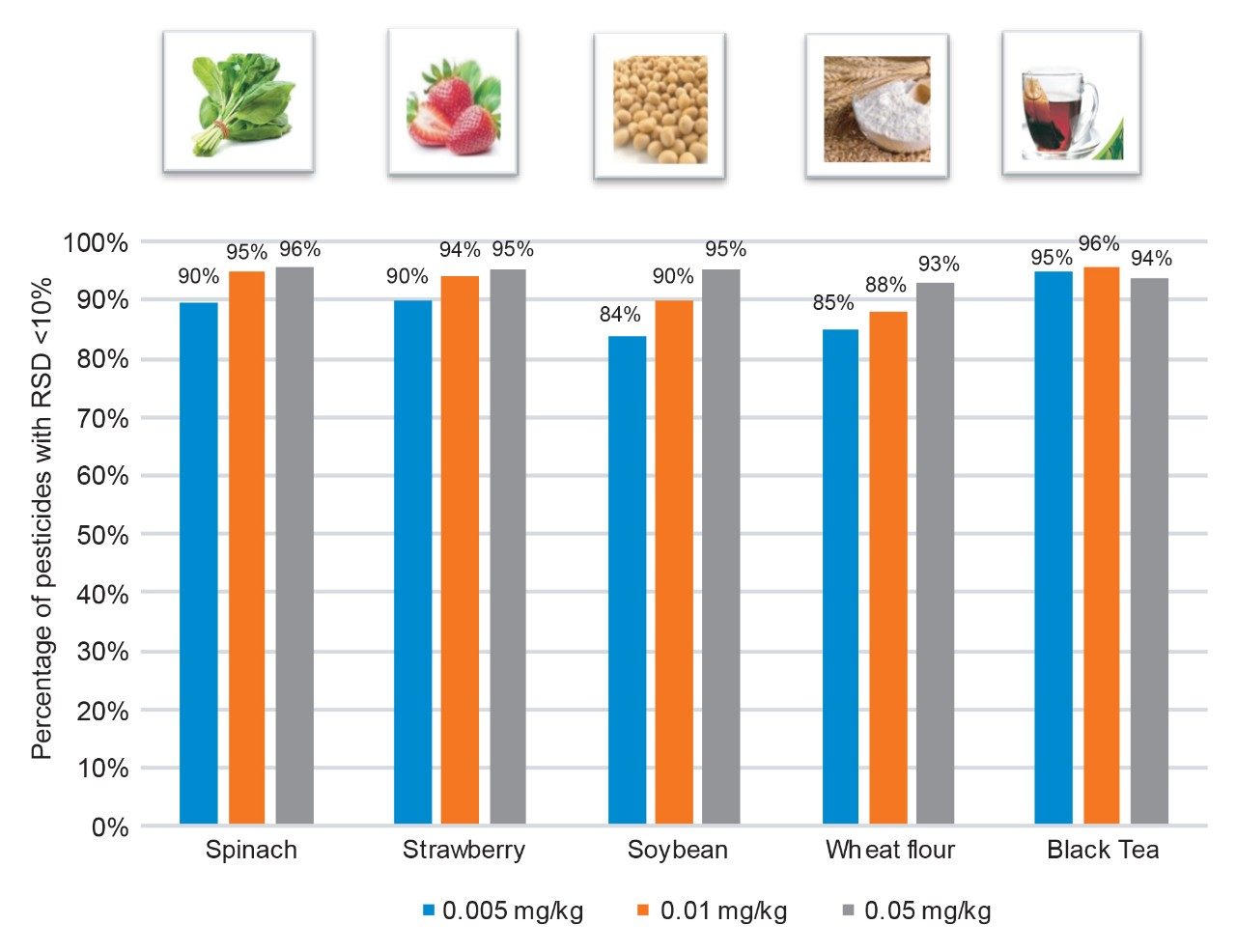
The precision of the LC-MS/MS measurements was calculated for the 256 representative pesticides from the replicate (n=6) determination of matrix-matched standards at three concentrations (0.005, 0.01, and 0.05 mg/kg). The precision of the measurement was good with more than 85% of the detected pesticides exhibiting RSDs for peak area of less than 10% (see Figure 6).
Matrix effects for the 256 compounds in the various matrices were calculated by comparing the ratio of the slope of the matrix-matched calibration graph for each commodity to that of the solvent calibration graph. Figure 7 summarizes the range of matrix effects observed for each commodity. All commodities show some degree of matrix suppression (response suppressed by >20%) and enhancement (response of the analyte increased by >20%) so matrix-matched calibration is recommended. Procedural calibration or standard addition are alternative approaches which compensate for matrix effects and recovery losses.
In order to investigate matrix effects further, soybean samples were prepared using QuEChERS and the extracts diluted (5x and 10x). Matrix-matched calibrants were prepared to 0.02 mg/kg in both crude and diluted extracts and analyzed. To investigate the impact of co-extractives, full scan RADAR acquisition was enabled alongside the MRM transitions for the 552 compounds of interest. Figure 8 shows an overlay of the total ion current (TIC) chromatograms from RADAR for the various soybean extracts. There is a significant peak observed at 3.9 minute in the crude extract trace (blue). One compound of interest, carbofuran 3-hydroxy, also elutes at 3.9 minute in the chromatogram based on this LC gradient.
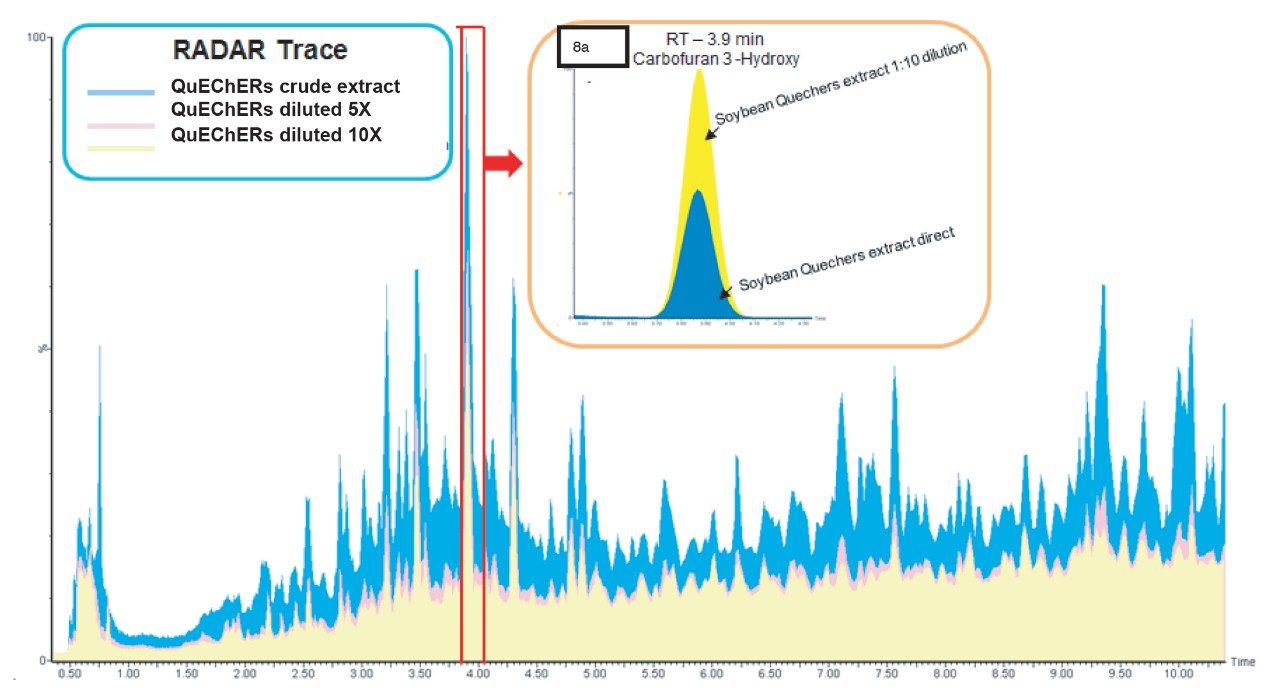
The presence of co-eluting matrix co-extractives can cause ion enhancement/suppression or isobaric interference. As can be seen in inset 8a, the peak area of carbofuran 3-hydroxy is almost doubled when the QuEChERS crude extract of soybean is diluted 10 times (yellow) compared to the crude QuEChERS extract (blue). The dilution of these QuEChERS crude extracts (as shown in Figure 8) reduces the matrix effect while still achieving the required LOD, without the need for sample cleanup. Dilution also reduces the loading of co-extractives into the system, increasing UPLC column longevity, decreasing the frequency of routine instrument maintenance and improving analytical productivity.
Identification criteria, retention times and ion ratios, were calculated and flagged using TargetLynx XS. The retention time and ion ratio of each analyte detected in the sample should correspond to that of the calibration standard reference.4 The retention times of all 256 representative pesticides, in all the matrices studied, were found to be within the tolerance of ±0.1 min. The ion ratios from the analysis of matrix-matched calibrants were within ±30% of the reference values for all 256 compounds.
720006886, Revised February 2021