For research use only. Not for use in diagnostic procedures.
This application note describes a clinical research method for the extraction and analysis of E2 and E1 from serum, using Waters ACQUITY UPLC I-Class System using a CORTECS Phenyl Column followed by mass detection using a Xevo TQ-XS Mass Spectrometer
The two major biologically active estrogens in non-pregnant humans are 17 â-estradiol (E2) and estrone (E1). E2 is produced primarily in the ovaries and testes by the aromatization of testosterone, whereas, the majority of E1 is derived from androstenedione. E2 can be metabolized to E1 and conversion of E1 to E2 is also possible, making the measurement of both compounds desirable.
The greatest challenge when analyzing E2 and E1 is the requirement to measure down to low concentration levels for certain clinical research applications. Currently, some immunoassay techniques lack analytical sensitivity and more commonly selectivity, while published LC-MS/MS methods use large sample volumes with complex sample extraction, often including derivatization.
Here we describe a clinical research method for the extraction and analysis of E2 and E1 from serum. Chromatographic separation of extracted samples was performed with a Waters ACQUITY UPLC I-Class System using a CORTECS Phenyl Column followed by mass detection using a Xevo TQ-XS Mass Spectrometer (Figure 1).

|
System: |
ACQUITY UPLC I-Class (FTN) with Column Manager |
|
Needle: |
30 μL |
|
Column: |
CORTECS Phenyl 2.7 μm, 2.1 × 50 mm (P/N 186008319) |
|
Mobile phase A: |
Aqueous 0.05 mM Ammonium fluoride |
|
Mobile phase B: |
Methanol |
|
Needle wash solvent: |
80% Methanol(aq) |
|
Purge solvent: |
10% Methanol(aq) |
|
Column temp.: |
50 °C |
|
Injection volume: |
20 μL |
|
Flow rate: |
0.3 mL/min |
|
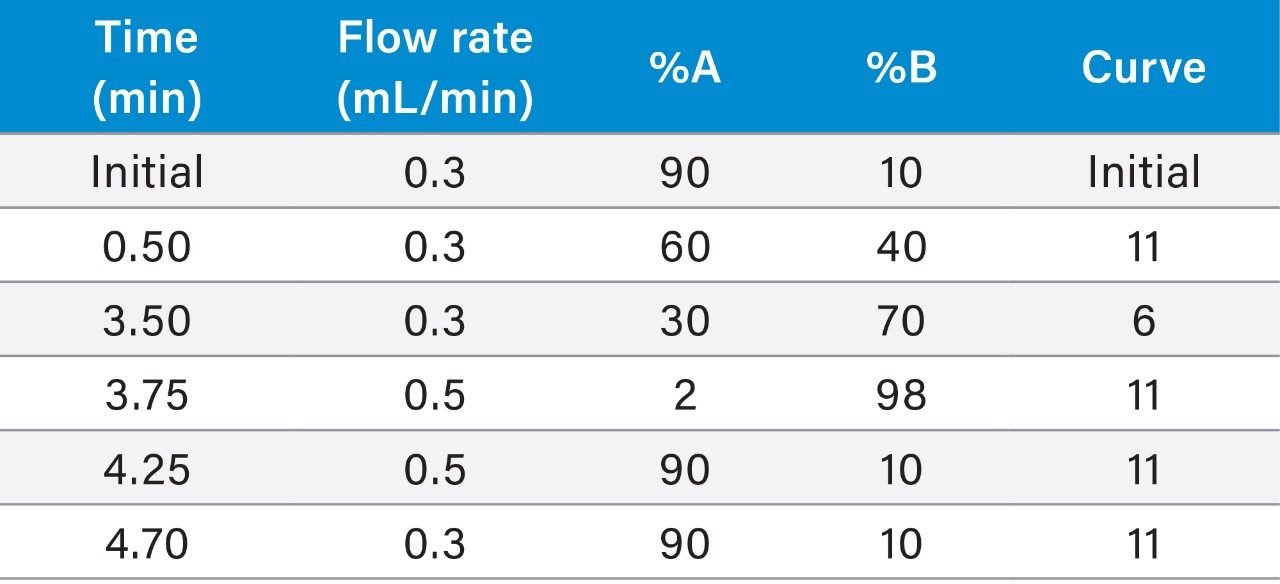
Gradient: |
See Table 1 |
|
Run time: |
4.75 min |
|
System |
Xevo TQ-XS |
|
Resolution |
MS1 (0.7 FWHM) MS2 (0.7 FWHM) |
|
Acquisition mode: |
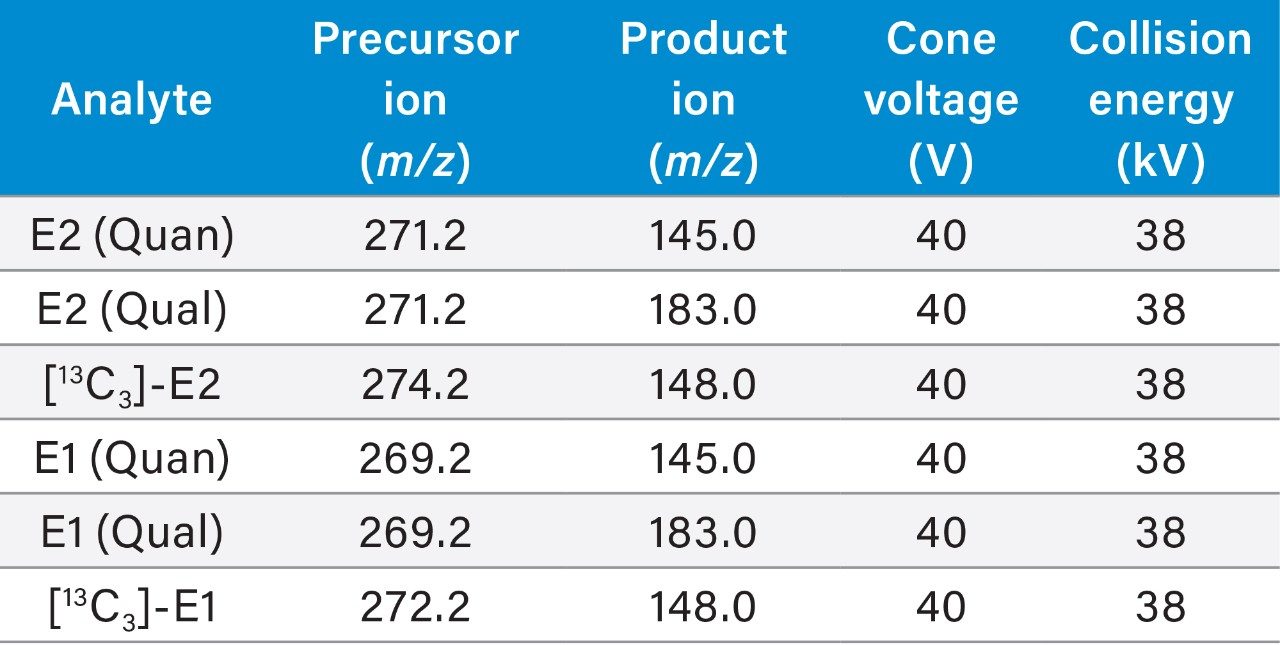
Multiple Reaction Monitoring (MRM) (See Table 2 for details) |
|
Polarity |
ESI - |
|
Capillary |
2.2 kV |
|
Source temp. |
150 °C |
|
Desolvation temp. |
600 °C |
|
Dwell time |
0.05 s |
MassLynx v4.2 with TargetLynx XS Application Manager
Operating backpressure at the initial conditions is ~3000 psi.
E2 and E1 certified reference solutions and their stable labeled internal standards (13C3) were purchased from Cerilliant (Round Rock, TX). Calibrators were prepared in a surrogate matrix of MSG4000 stripped serum purchased from Golden West Biologicals (Temecula, CA). The calibration range for E2 was 3–1000 pg/mL (~11.1–3700 pmol/L) and 2–1000 pg/mL (~7.4–3700 pmol/L) for E1. The QC samples were prepared in a surrogate matrix of MSG4000 stripped human serum purchased from Golden West Biologicals (Temecula, CA) at ~10 pg/mL, ~75 pg/mL, and ~750 pg/mL (~37 pmol/L, ~275 pmol/L, and ~2750 pmol/L). Distilled water was prepared in-house using a water purification system by ELGA (High Wycombe, UK). Methanol and ethyl acetate were purchased from Honeywell (Bracknell, UK). Ammonium fluoride and hexane were purchased from Sigma-Aldrich (Gillingham, UK).
Note: To convert conventional mass units (pg/mL) to SI units (pmol/L), multiply by 3.671 for E2 and 3.699 for E1.
To 250 μL of sample, 20 μL of internal standard (~2 ng/mL of [13C3]-E2 and [13C3]-E1) was added and mixed. Liquid/liquid extraction was performed by adding 1 mL of 85:15 (v:v) hexane:ethyl acetate, mixing thoroughly for ten minutes. Samples were centrifuged at 4000 g for five minutes prior to 700 μL of the top organic layer being transferred into a 96-well plate containing 1 mL glass inserts (P/N: 186000855). Samples were evaporated to dryness and reconstituted in 20 μL of methanol followed by 30 μL of distilled water.
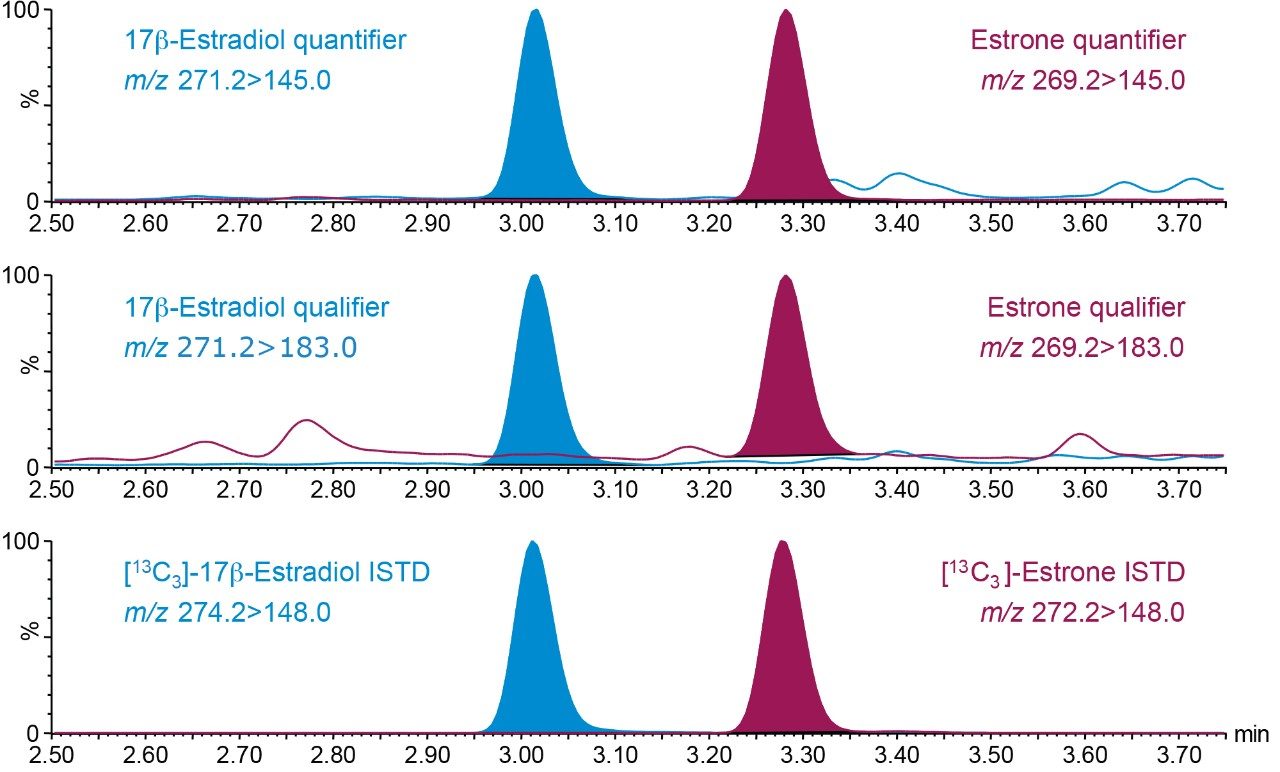
Chromatograms of E2 and E1 from a human serum sample are shown in Figure 2.
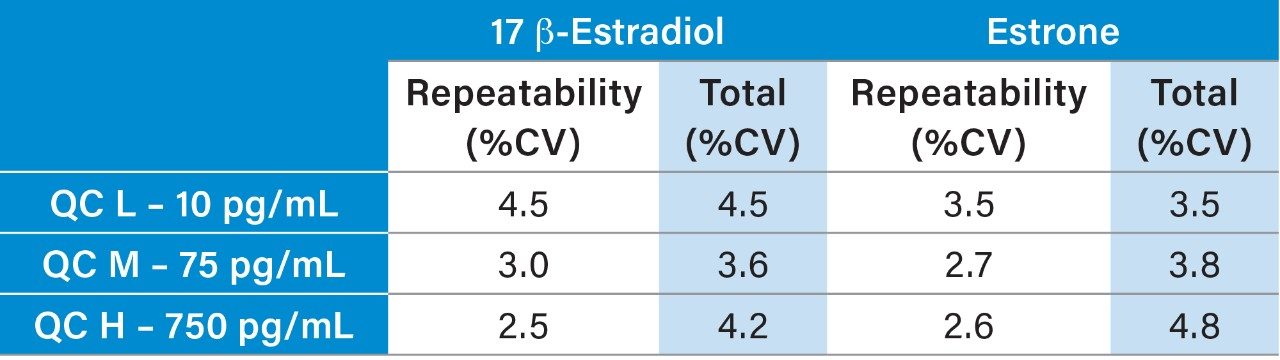
Precision was assessed by extracting and measuring five replicates of samples across five days. Total precision and repeatability were ≤4.8% CV at concentrations between 10 and 750 pg/mL (37 and 2775 pmol/L) for both E2 and E1 (Table 3).
Analytical sensitivity was assessed by extracting and quantifying five replicates of low level E2 and E1 samples prepared in stripped serum over five days. The LLoQ was determined to be the lowest concentration at which precision (repeatability) was ≤20%CV and S:N(ptp) was ≥10:1, with no significant carryover observed when tested at 2000 pg/mL. The LLoQ for E2 was determined to be 3 pg/mL (11 pmol/L) (Table 4 and Figure 3) and 2 pg/mL (7.4 pmol/L) for E1.
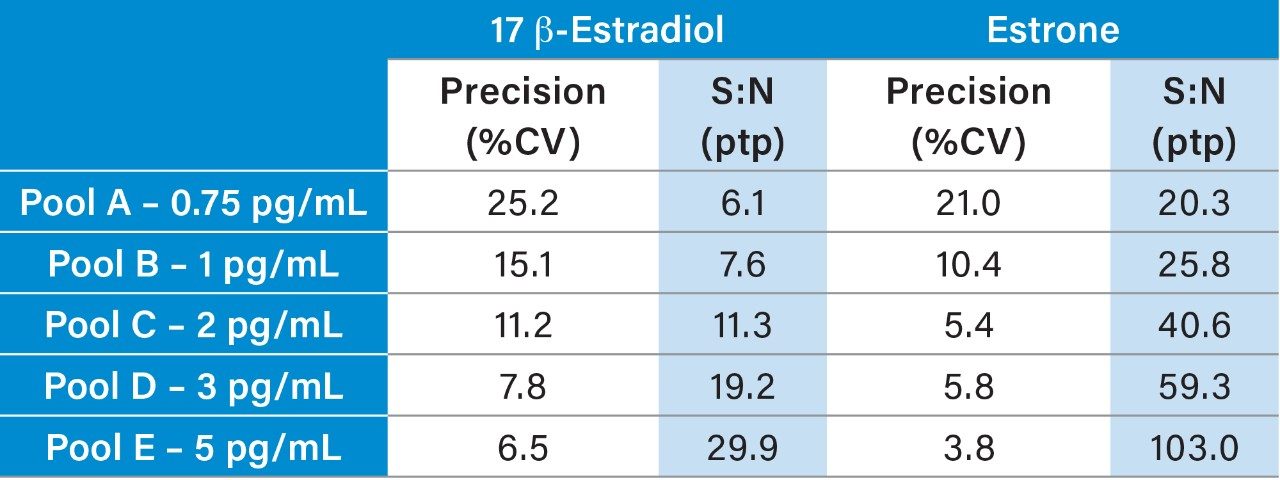
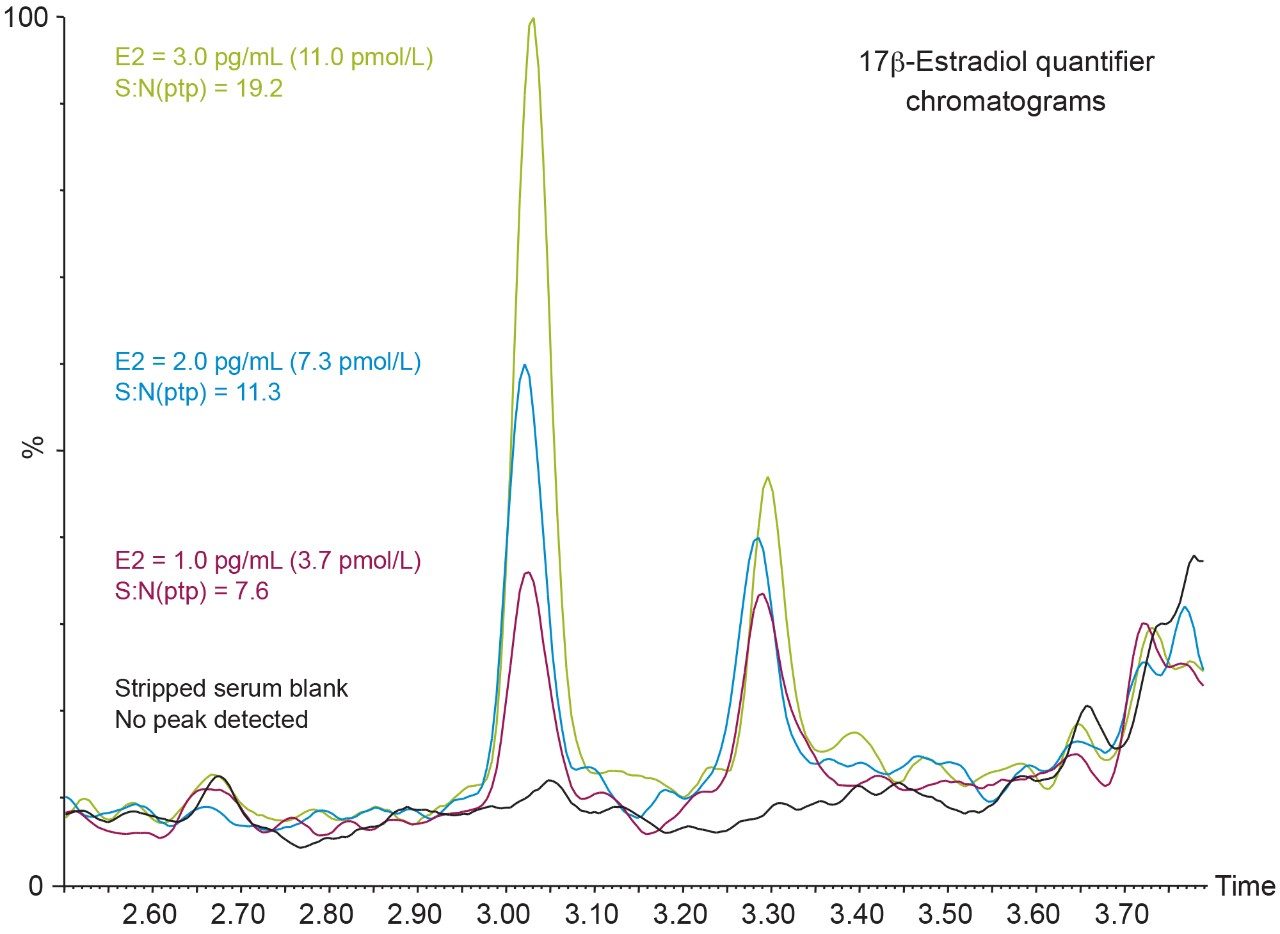
Figure 3 shows typical chromatograms of a blank and low level samples for E2. While an LLoQ of 1 pg/mL (3.7 pmol/L) was not achieved, a blank stripped serum sample can be differentiated from the same serum spiked at 1 pg/mL (3.7 pmol/L) of E2. Figure 3 also shows the importance of chromatographic resolution of E1 from E2, where the isotopic contribution of E1 is observed in the E2 MRM transition.
The method was shown to be linear across the range of 0.434–1117 pg/mL (1.59–4099 pmol/L) for E2 and 0.647–1113 pg/mL (2.39–4118 pmol/L) for E1, when low and high pools were mixed in known ratios to give ten samples over the range. All calibration lines in spiked stripped serum were linear with a coefficient of determination (r2) >0.998 across ten separate occasions for E2 and E1.
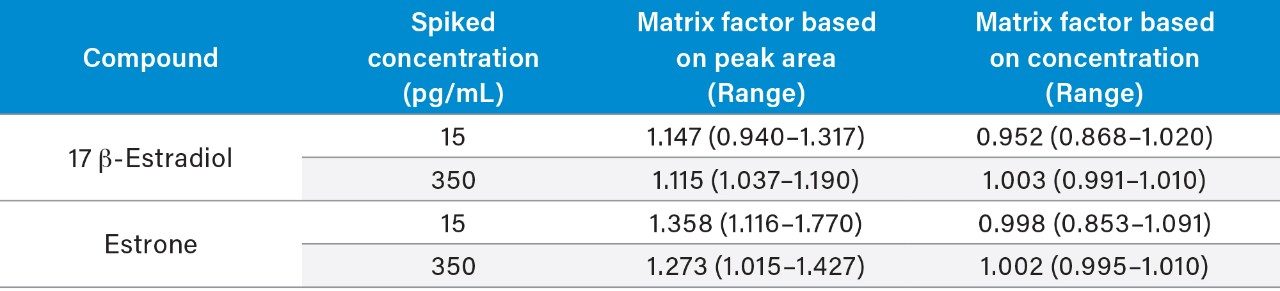
Typical endogenous interferences (albumin, bilirubin, cholesterol, intralipid, triglycerides, and uric acid) were tested and %recoveries of the test samples compared to controls were all within ±15%. Matrix effect investigations were performed using donor serum samples from six individuals. The endogenous peak areas were separately quantified and post-spiked samples at low and high concentration levels were adjusted using the mean peak areas to enable comparison to solvent spiked samples. While some variability in the matrix factor was observed when looking at the peak areas, these were compensated for by the internal standard (Table 5).
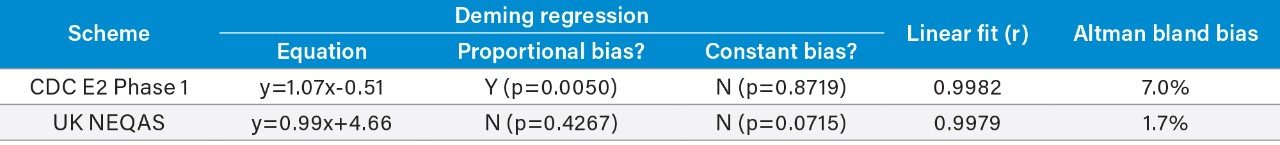
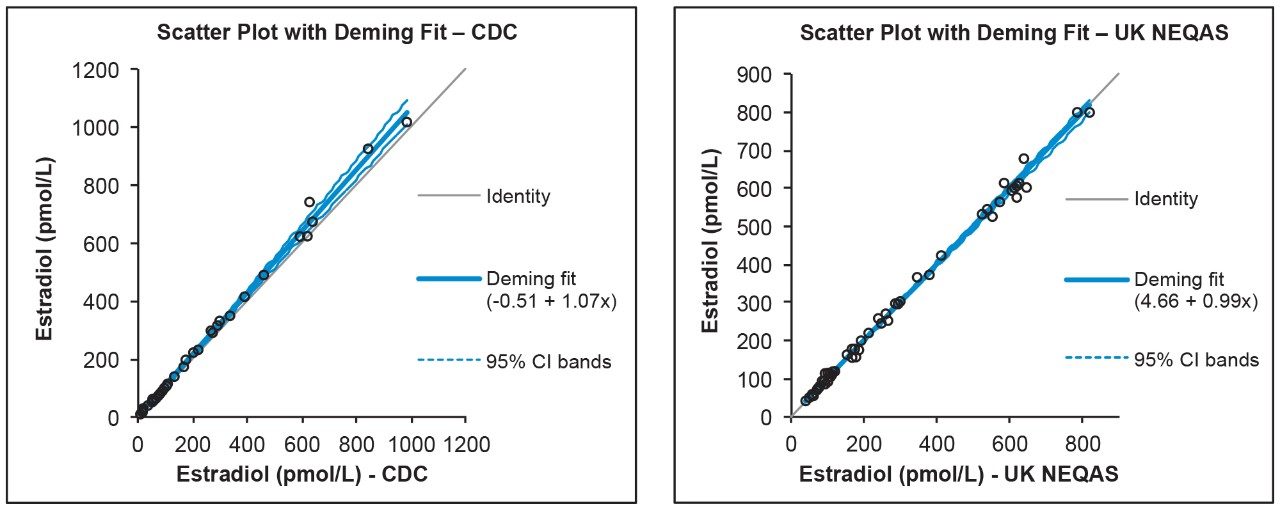
Accuracy was assessed by analyzing 40 CDC E2 Phase 1 samples and 53 UK NEQAS E2 samples with calculated concentrations compared to the assigned values. The correlation for E2 can be seen in Table 6 and Figure 4, showing excellent agreement with both EQA schemes. BCR certified reference material samples at concentrations of 31, 188, and 365 pg/mL (114, 690, and 1340 pmol/L) were also extracted in triplicate and analyzed, with all measurements being within ±4.9% of their assigned values for E2.
A clinical research method has been developed with UPLC-MS/MS for the analysis of E2 and E1 in serum with good analytical sensitivity. Using only 250 μL of serum, this method was able to distinguish between a blank stripped serum sample and the same matrix spiked with 1 pg/mL of E2, without the use of derivatization.
The assay described demonstrates excellent precision over five days, accuracy, and linearity across the measuring range with no significant interference from the endogenous compounds tested with minimal matrix effects observed.
720006315, June 2018